Abstract
Background
Patient leukapheresis material collected for tisagenlecleucel manufacture can be cryopreserved for up to 30 months prior to use in manufacturing, allowing potential benefits in scheduling, shipping, and patient management (Qayed et al, Cytotherapy 2022). Recent data have reported leukapheresis in patients with DLBCL after failure to first line of therapy and the positive impacts of collecting these patients early on the quality of the leukapheresis material (Grisariu et al, EBMT 2022; Farina et al, EBMT 2022). Here, we evaluated the prevalence of tisagenlecleucel orders manufactured from leukapheresis material collected prior to order submission (historical collections), assessed whether this prevalence was similar across countries and indications, and reported the impacts of using historical collections on tisagenlecleucel manufacturing outcomes.
Methods
Between Jan-2020 and Jun-2022, over 5000 patients with ALL or DLBCL globally had cryopreserved leukapheresis material available for commercial tisagenlecleucel manufacture. Date of order submission for tisagenlecleucel manufacture, leukapheresis collection, and pickup of the cryopreserved leukapheresis product from the treatment center was captured through CellChain™, the Novartis online portal for order management. Leukapheresis material was defined as a historical collection if the date of leukapheresis occurred before the date of order submission. Prevalence of historical collections between the US, Europe, and other countries where tisagenlecleucel is commercially available was analyzed. Manufacturing success rates (MSR) and time between order submission to pickup of the cryopreserved leukapheresis material were evaluated.
Results
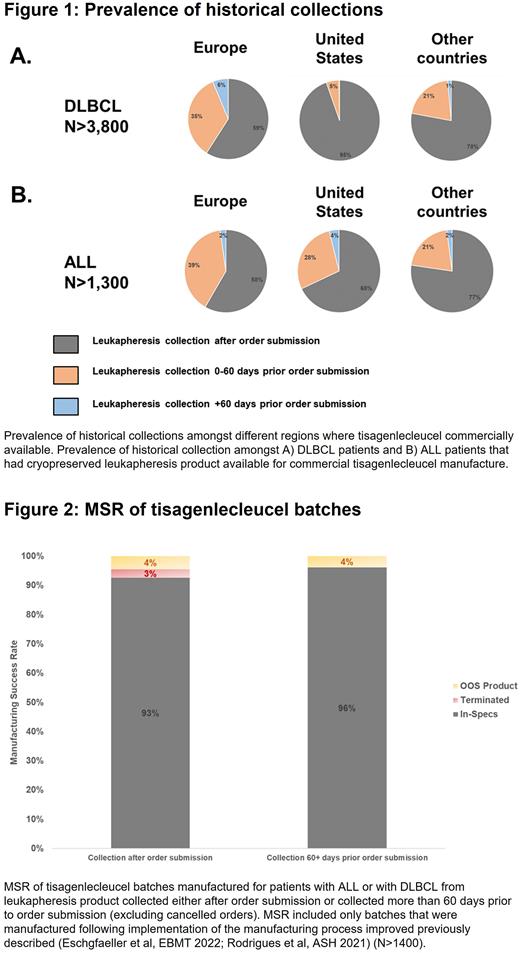
Treatment center practices on when leukapheresis was performed relative to order submission were significantly different between countries and indication. Amongst patients with DLBCL, the percentage of patients where leukapheresis occurred prior to order submission was 41% in Europe, 5% in the US, and 22% in other countries. Further, the percentage of patients with DLBCL collected more than 60 days prior to order submission was 6% in Europe, less than 0.5% in the US, and 1% in other countries (Fig. 1A). For patients with ALL, differences in percentage of historical collections were less striking: 42% in Europe, 32% in the US, and 23% in other countries. Interestingly, the percentage of patients with ALL collected more than 60 days prior to order submission was the highest in the US at 4%, vs. 2% in Europe or other countries (Fig. 1B).
Compared to batches manufactured from collections performed after order submission, batches manufactured from historical collections showed a reduced median time between order submission and pickup of the cryopreserved leukapheresis material from 12 days to 5 days. Compared to products manufactured from leukapheresis material collected after order submission, a marginally higher tisagenlecleucel MSR was observed when the leukapheresis material was collected more than 60 days prior to order submission (96% MSR vs. 93% MSR), with no manufacturing terminations reported (Fig. 2).
Conclusions
This report describes the real-world experience of when patients are collected compared to when the order for tisagenlecleucel manufacture is submitted. Having a leukapheresis material already collected and cryopreserved at time of order submission, decreased the median time between order submission and pickup of the leukapheresis product from 12 to 5 days, potentially leading to faster patient access to tisagenlecleucel. A small but significant number of patients intended for tisagenlecleucel treatment were collected more than 60 days prior order submission, suggesting that these patients may have been collected prior to their eligibility for CAR-T. However, this practice to collect patients pre-emptively, especially more than 60 days prior order submission, was highly variable and was most prevalent in Europe for patients with DLBCL at 6% of patients and most prevalent in the US for patients with ALL at 4% of patients. A marginally higher MSR when using a leukapheresis material collected more than 60 days prior order submission was observed. However, the numbers were small and thus the benefits of early collected leukapheresis material on the manufacturing outcomes remain to be confirmed in larger sample size.
Disclosures
Smrekar:Lek Pharmaceuticals, Ljubljana, Slovenia: Current Employment. Vendramin:Novartis Farma SpA, Origgio VA, Italy: Current Employment. Fernandez:Novartis Pharmaceuticals, Macquarie Park, NSW, Australia: Current Employment. Eschgfaeller:Novartis Pharma AG, Basel, Switzerland: Current Employment. Fong:Novartis Pharmaceuticals Canada Inc., Dorval, QC, Canada: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal